The Avogadro Number
- The mole is a unit of measure for the amount of a chemical substance. The mole is equal to Avogadro's numb.
- But if we consider a weight of substance that is the same as its formula (molecular) weight expressed in grams, we have only one number to know: Avogadro's number, 6.022141527 × 10 23, usually designated by NA. Amadeo Avogadro (1766-1856) never knew his own number!

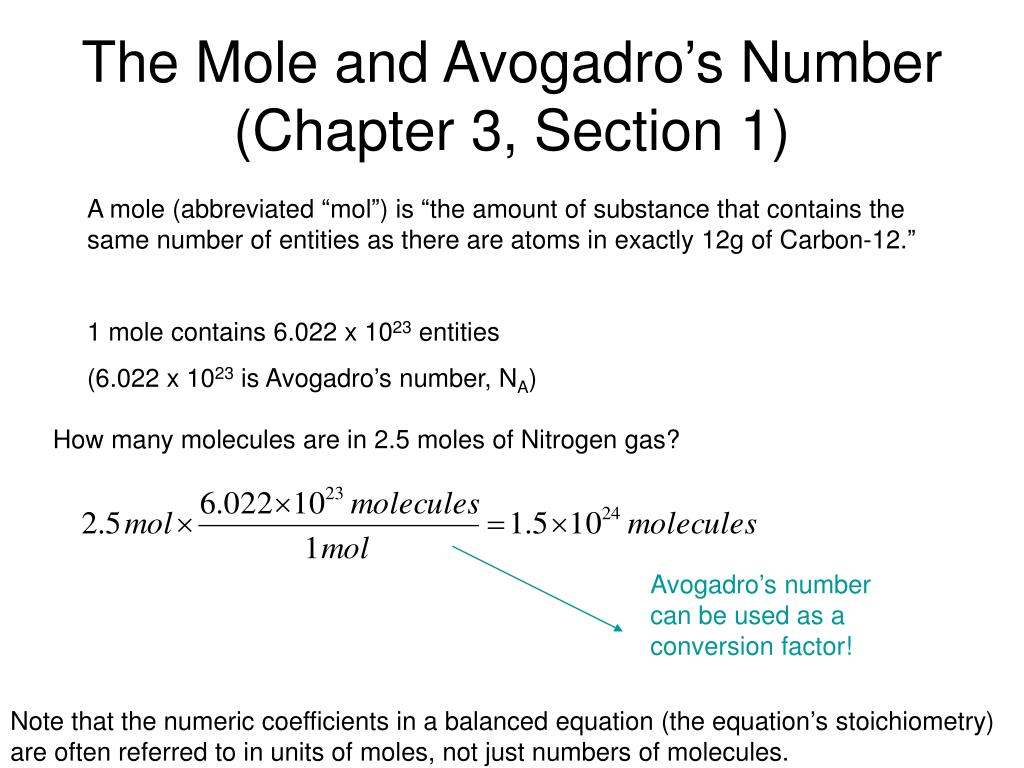
Avogadro's number, or Avogadro's constant, is the number of particles found in one mole of a substance. It is the number of atoms in exactly 12 grams of carbon -12. This experimentally determined value is approximately 6.0221 x 10 23 particles per mole. The Avogadro constant is named after the Italian scientist Amedeo Avogadro (1776–1856), who, in 1811, first proposed that the volume of a gas (at a given pressure and temperature) is proportional to the number of atoms or molecules regardless of the nature of the gas. Since Avogadro's number is 6.022 × 10 23, it only makes sense that the holiday starts at 6:02 a.m. Revelers tell chemistry jokes, blow bubbles of natural gas that they set ablaze, toast with drinks chilled by dry ice and even recite the mole pledge of allegiance.
The currently accepted formal definition of a mole is the number of carbon-12 atoms in exactly 12 grams of the pure substace. This is not a good operational definition, however, because it takes too long to find, purify, and count all those atoms. The best experimental value is based on x-ray diffraction experiments on silicon crystals and puts the number within 0.0000010 of 6.0221415 x 10^23. However, that number is not satisfactory to the perceptive (read 'smart aleck') student of chemistry who challenges his teacher with the fact that the definition requires that Avogadro's number be an integer. Because the definition of a (kilo)gram, represented by a platinum- iridium cylinder stored in Severes, France, changes slightly with calibration experiments and cleaning, the size of the mole theoretically changes proportionally. Fox and Hill, two noun-named professors at Georgia Tech, propose to define the kilogram in terms of a fixed value of Avogadro's number. Perhaps you and your students can determine why they chose the value they propose (602,214,141,070,409,084,099,072). Once you have defined NA, then the kilogram is determined and you can tell the keepers of the kilogram cylinder that their work is done.

The Mole Avogadro's Number And Stoichiometry

Using The Avogadro Number

